
Me as a Teacher and Mentor:
I have been passionate about teaching and mentoring since the very beginning of my academic journey. My first experiences came as a BSc honours student, when I was invited to deliver guest lectures in introductory ecology courses—and I was immediately hooked. That enthusiasm has never faded; I continue to find deep joy in guiding students through subjects I care about most, including genetics, ecology, community-engagement and research ethics.
Over the years, I have devoted considerable effort to developing my teaching and mentoring practice (see below for specific steps I have taken). I earned a Certificate in Teaching from the Centre for Innovation in Teaching and Learning at the University of Illinois Urbana–Champaign and have been recognised multiple times on the university’s list of “Teachers Ranked as Excellent by Their Students.”
My teaching philosophy centres on cultivating inclusive, creative, and active learning environments where students feel empowered to grow, explore, and contribute.

My Teaching Philosophy
I believe teaching as a skill should be adaptive, and teaching strategies should be principally based on the needs of the students. What works for some classes/students are not always going to work for others – teaching should not have a one-shoe-fits-all approach. I therefore encourage regular assessments though quarterly feedback, and I also provide students with the opportunity to anonymously make recommendations or suggestions on how classes could be improved. In past courses that I have taught, this approach has been especially useful to gauge topics or activities with which students require additional help, but has also provided insights into activities that promote learning and engagement.
I believe in setting clearly defined expectations, and keeping students to them. Defining expectations at the start of the course, and reiterating your expectations for each topic, provides a manageable curriculum to both instructors and students.
I believe that successful students are creative scientists. I gear my course outline and assignments to follow the principles of Bloom’s taxonomy. My lecture planning and assessments are set up to encourage students to achieve the desired level of teaching (from remembering, to understanding, applying, analyzing, evaluating, and ultimately creatively thinking about science). For example, at the beginning of each class period, I’ll review previous lecture material through quick question discussions that bridges previous topics to the next lesson. This allows me to assess areas in the learning process where students might require additional support. My ultimate goal for individual topics, and for each course, is to achieve the pinnacle of Bloom’s taxonomy where students can design, assemble, construct, develop and investigate topics related to the class either through practical application in class or at home and in their future careers. Providing students with toolsets that allow them to creatively interpret topics in Biological Sciences is my ultimate goal.
I believe in promoting inclusive, diverse learning environments. Now more than ever, there is a need to increase inclusivity and promote diversity in classrooms. My background as an international student and teacher has encouraged me to set up actionable practices that promote inclusivity and diversity. For example, I am part of a graduate and early career faculty group that has created teaching resources for diversifying and decolonizing syllabi in Life and Environmental Sciences (see my DEIA in STEM page). We have developed a list of college-level resources (articles, podcasts, videos, etc) that can be used as teaching tools in undergraduate classrooms. These resources may, for example, cover topics of racial justice, traditional ecological knowledge, environmental racism, and other related topics. In my classroom I work to develop awareness of current and historical injustices in science, and I am conscientious of different backgrounds and viewpoints that students may have. I promote a class culture that reflects the merits of my own prospective lab: be respectful, be honest, be supportive.
Supervision and training of undergraduate & graduate students
*Students that I trained/mentored, with whom I have co-authored publications
-
Brian Graves, (graduate student, training in molecular laboratory and genetic analysis at the Carl R. Woese Institute for Genomic Biology, 2023, University of Illinois)
-
Ariane Thomas*, Department of Anthropology, University of Iowa (graduate student, training in molecular laboratory and genetic analysis, training in ancient DNA molecular techniques at the Carl R. Woese Institute for Genomic Biology, 2020, 2023, University of Illinois)
-
Vivian Cheng (graduate student, training in molecular laboratory and genetic analysis, training in ancient DNA molecular techniques at the Carl R. Woese Institute for Genomic Biology, 2021 – present, University of Illinois)
Sahara Vilchis & I doing elephant research Ariane Thomas & I doing ancient DNAresearch
(photos used with permission)
-
Sahara Vilchis* (graduate student, training in molecular laboratory and genetic analysis, training in ancient DNA molecular techniques at the Carl R. Woese Institute for Genomic Biology, 2021 – present, University of Illinois)
-
Dr. Karthik Yarlagadda* (graduate student, training in ancient DNA molecular techniques at the Carl R. Woese Institute for Genomic Biology, 2018 – University of Illinois)
-
Aimée Carbaugh (graduate student, training in ancient DNA molecular techniques at the Carl R. Woese Institute for Genomic Biology, 2018 – University of Illinois)
-
Cassidy Donnelly* (undergraduate student, training in molecular laboratory and genetic analysis methodologies, 2017 & 2018 – University of Illinois)
-
Maria Cox (graduate student, training in ancient DNA molecular techniques at the Carl R. Woese Institute for Genomic Biology, 2017 – University of Illinois)
-
Puseletso Motsomane (undergraduate & graduate student, training in molecular laboratory techniques, 2014 & 2015 – University of Pretoria)
-
Ali Coleman (graduate student, co-supervision of BSc. (hons) Zoology thesis project, 2014)
-
Teagan Carpenter-Kling (undergraduate student, training in molecular laboratory techniques, 2012 – University of Pretoria)
-
Jessica Humphreys (undergraduate student, training in molecular laboratory techniques, 2011 – University of Pretoria)


Example course that I conceptualized, developed and taught
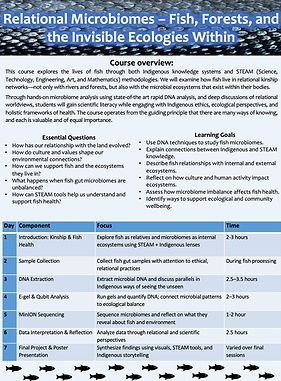
Relational Microbiomes - Fish, Forests, and the Invisible Ecologies Within
Syllabus:
Instructors: Dr. Alida de Flamingh
(in collaboration with SeaAlaska Heritage Institute (images copyright)
and University of Alaska SouthEast - 3 credit course
Class folder: https://go.illinois.edu/Relational_Microbiomes_Course
Course Overview
This course explores the lives of fish through both Indigenous knowledge systems and STEAM (Science, Technology, Engineering, Art, and Mathematics) methodologies. We will examine how fish live in relational kinship networks—not only with rivers and forests, but also with the microbial ecosystems that exist within their bodies.
Through hands-on microbiome analysis and deep discussions of relational worldviews, students will gain scientific literacy while engaging with Indigenous ethics, ecological perspectives, and holistic frameworks of health. The course operates from the guiding principle that there are many ways of knowing, and each is valuable and of equal importance.
Students will conduct DNA-based microbiome analysis and reflect on the invisible relationships that sustain life internally and externally. Indigenous knowledge frames these relationships through kinship, care, and responsibility, providing context for understanding not just what microbes are present, but why those relationships matter.
Essential Questions
-
How has our relationship with the land changed over time?
-
How do culture and values affect our relationship with the land and environment?
-
How can you support the health of fish and the ecosystem (forests and rivers) that they live in? What are some things/activities that you can do?
-
What impacts can unbalanced gut microbiomes have on fish health?
-
How can DNA analysis and other STEAM tools help us understand and support fish and fish microbiome health?
Learning Goals
-
Apply molecular biology techniques (DNA extraction, sequencing, analysis) to study fish microbiomes.
-
Articulate how Indigenous knowledge and STEAM disciplines intersect and inform one another.
-
Understand and explain how fish live in reciprocal relationship with their environments—both internal (microbial) and external (ecological).
-
Reflect on how cultural values and human activity affect ecosystems and the health of aquatic life.
-
Evaluate how unbalanced microbiomes can impact fish behavior, immunity, and survival.
-
Explore actions individuals and communities can take to care for and support ecosystems and kin species.



Teaching Experience
-
Visiting Instructor or record (Summer 2025) Indigenous Summer STEAM Camp – Relational Microbiomes: Fish, Forests, and the Invisible Ecologies Within. I conceptualized, developed, and taught this course through collaboration with SeaAlaska Heritage Institute (SHI) and University of Alaska Southeast (3 credits; CRN 51584): https://go.illinois.edu/Relational_Microbiomes_Course
-
Guest Lecture (Spring 2023) VCM 540 Conservation and Ecosystem Health (topic: Conservation Genetics).
-
Instructor (Fall 2015, Spring & Fall 2016, Spring 2017): Instructor for the Animal Sciences class ANSC207, Companion Animal Care and Biology, University of Illinois at Urbana-Champaign
-
Teaching Assistant (Fall 2018) Animal Biology IB 104, dissection lab, School of Integrative Biology, University of Illinois at Urbana Champaign (CITL achievement - Teacher ranked as excellent by their students)
-
Teaching Assistant (Spring 2018) Environmental Biology IB 105, School of Integrative Biology, University of Illinois at Urbana Champaign
-
Teaching Assistant (Fall 2017) Animal Biology IB 104, dissection lab, School of Integrative Biology, University of Illinois at Urbana Champaign (CITL achievement - Teacher ranked as excellent by their students)
-
Guest Lecture (Spring 2022): ANSC 406 Population Genetics. Introduction to Paleogenomics
-
Guest Lecture (Spring 2021, 2022, 2023): NRES 407: Population Ecology. Conservation Genetics and Genomics
-
Guest Lecture (Spring 2017 & 2019): ANTH 247: Forensic Genetics. Conservation Genetics and Wildlife Forensics
-
Guest Lecture (Spring 2016) Why elephants don’t get cancer. 500-level seminar course, Southern Illinois University
-
Graduate WIS member (Fall 2015, Spring & Fall 2016) of the University of Illinois Woman in Science (WIS) club
-
Panel Member for Illini Wildlife and Conservation Club (February 2016): Discussion with undergraduate students about studies, research and teaching at a graduate level.
-
Guest Speaker (September 2012): Microsatellite workshop presented by the Forestry and Agricultural Biotechnology Institute, University of Pretoria: Landscape Genetics: uniting genetics and ecology through the use of microsatellites.
-
Guest Speaker (May 2012): Microsatellite workshop presented by the Forestry and Agricultural Biotechnology Institute, University of Pretoria: Microsatellite analysis of Degraded DNA: what to choose, what to use and what to do
-
Field research assistant (2011), assisting in small mammal trapping practical classes for the final year mammalogy students
Instructional courses attended to increase my teaching effectiveness
-
MakerGirl – Teaching with Technology (1hr, 10/05/2016), Centre for Innovation in Teaching and Learning (CITL), University of Illinois at Urbana-Champaign
-
Making the most out of discussions (1.5hr, 03/02/2017), CITL
-
Designing cooperative learning experiences (1.5hr, 03/17/2020), CITL
-
The power of presentations: Enhancing your slides for teaching engagement (1.5hr, 03/30/2020), CITL
-
Inclusive Lab Leaders (Fall 2019), a workshop seminar series that aims to boost trainee effectiveness. Topics include integrating diverse experiences and perspectives, navigating interpersonal challenges, and innovating new systems for mentoring and scientific advancement. 21st Century Scientists & The Beckman
-
Exploring Difference in the Biology Classroom: What Genetic Ancestry Tests Mean (and What They Don’t), Tuesday, 11 April 2023